Ort: Lise-Meitner-Hörsaal, Strudlhofgasse 4, 1. Stock
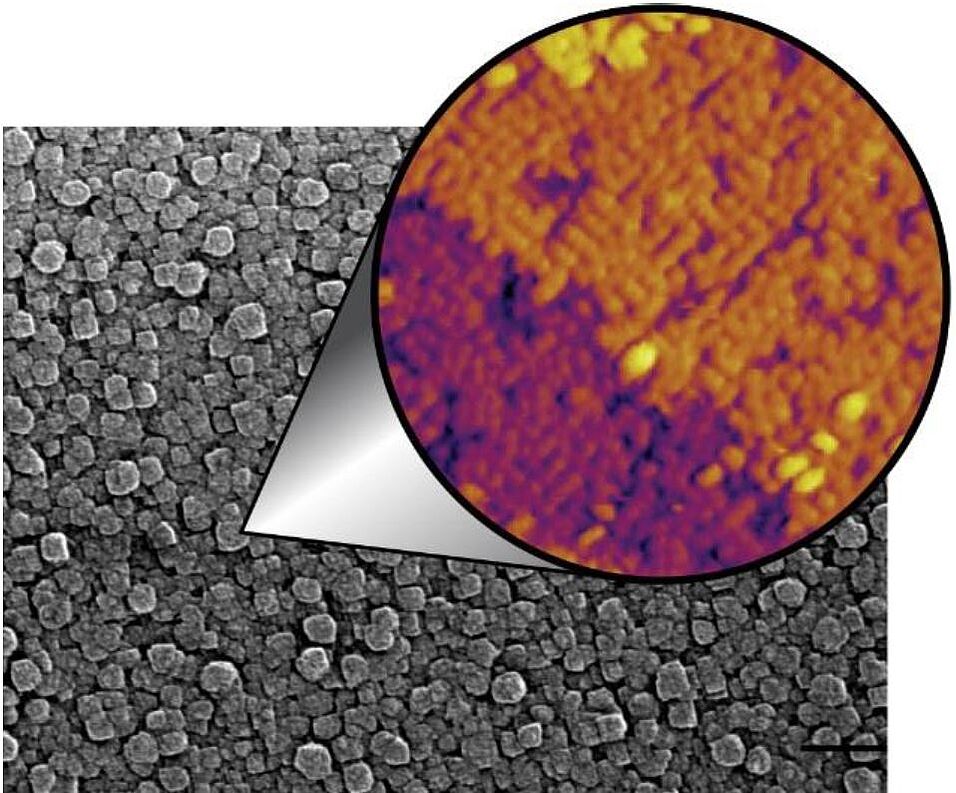
The arrangement of atoms on a catalyst surface controls its chemical reactivity. As a result, nanocatalysts with different shapes — and thus different surfaces — often have markedly different reactivities, which has led to the emergence of shape-controlled chemistry. Never-theless, predicting which faces, and thus which shapes, will be the most reactive under operating conditions has been hindered by our inability to image these tiny crystals at the atomic scale. I will show that shape-controlled crystal growth techniques can be used to produce highly aligned anatase TiO2 nanocrystals that can be studied at the atomic-scale with scanning tunneling microscopy and a panoply of other techniques. Despite being synthesized in solution, the surfaces of the nanocrystals are very clean and passivated by a protecting monolayer. Using these crystals, I will show that the most commonly used functionalization chemistry for oxide nanocatalysts, a carboxylic acid solution, causes the spontaneous reorganization of a reconstructed anatase nanocatalyst, leading to a five-fold increase in the number of reactive sites. Importantly, this restructuring does not occur when the nanocatalyst is exposed to carboxylic acids in ultrahigh vacuum, the typical environment for studying surface reactions. In addition, I will discuss the chemistry of a variety of near-atomically-perfect, self-assembled monolayers that can be prepared from solution on both anatase and rutile TiO2 surfaces.